Poster Presentation The Annual Scientific Meeting of the Endocrine Society of Australia and the Society for Reproductive Biology 2014
Comparison of four immunoassays for measurement of free Thyroxine (fT4) levels in pregnancy; which assay do you use? (#241)
Background: The aim of antithyroid drug treatment of hyperthyroidism due to Graves’ disease in pregnancy is to control maternal hyperthyroidism while minimizing risk of fetal hypothyroidism. Consequently, it is recommended in such pregnancies to maintain “free T4 (fT4) values at or just above the upper limit of normal” using trimester-specific fT4 values,” or in their absence to use “reference ranges for non-pregnant patients”1.
Aim: We sought to determine trimester-specific fT4 reference intervals (RI) for common methods used in Australia.
Method: Healthy, thyroid-peroxidase antibody negative women with singleton pregnancy ≤13 weeks gestation were followed prospectively throughout pregnancy with samples collected at Trimester-1 (T1), Trimester-2 (T2), Trimester-3 (T3), and postpartum (PP). Serum fT4 was measured by four immunoassays: Beckman DXI800, Roche e602, Abbott Architect and Siemens Centaur. Reference intervals are presented as 95%CI (calculated as mean±2SD). Differences in mean were tested using ANOVA.
Results: Samples from the same group of women were analysed by all four methods. There were 118 women at T1 (11.8±0.2) (mean±SE weeks gestation), 78 at T2 (24.4±0.3), 63 at T3 (35.9±0.3) and 73 at postpartum (PP).
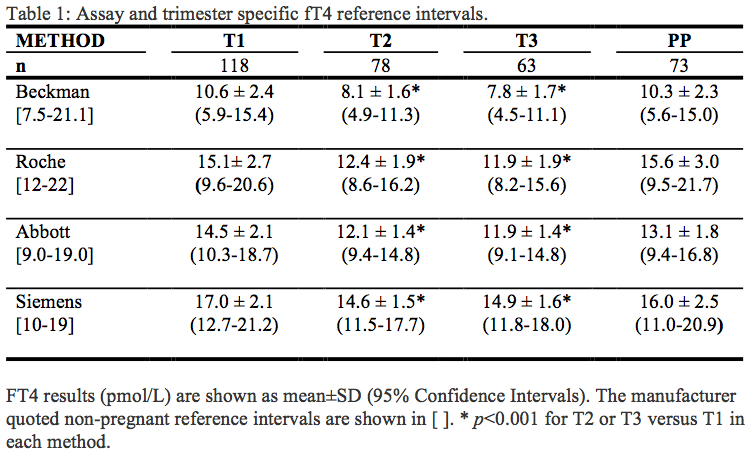
Within each method, all showed an approximate 15-20% decrease in fT4 in T2 and T3 compared to T1. Between methods, significant differences were present at all time points. The Beckman method was approximately 30% lower than the Roche and Abbott methods, and 45% lower than the Siemens method. Roche and Abbott gave similar results.
Conclusion: Both gestation and assay method had a significant influence on fT4 results in pregnant women. Knowledge of trimester and method specific reference intervals assists in optimizing antithyroid drug therapy in pregnancies affected by Graves’ disease.
- Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum. Thyroid. 2011;21(10):1081-25.